Introduction of stainless steel
Quickview:
Group of stainless steel (Inox) formed from alloy steel them, was originated in 1913 in Sheffield, England; Harry Brearley was trying a number of alloys to steel-barreled gun, and he noticed some rust sample cut not see reality and real seasoned difficult
Quickview:
Group of stainless steel (Inox) formed from alloy steel them, was originated in 1913 in Sheffield, England; Harry Brearley was trying a number of alloys to steel-barreled gun, and he noticed some rust sample cut not see reality and real seasoned difficult. When Mr. learn the depth of this amazing material, that contains 13% Cr steel. This result led to the development of stainless steel cutlery and also from here, Sheffield became famous. Random development works have also been performed in France at that time had made the summit of their development austenitic stainless steel first.
Stainless steel consumption in the world is increasing. It is the growth in demand for building and construction industry - where stainless steel is used to make decorative and rust, low maintenance requirements and durability. Many other industries are adopting stainless steel for the above reasons as well as it does not have many demands on the handling of the product, coating being brought to use, except that its price is higher than steel Carbon generally. Demonstrated the broad application that manufactures home appliances, they are growing provider of products, traditionally known as "white-whitegoods goods", are manufactured from stainless steel
They stainless steel
Stainless steels are iron base alloys that contain a minimum of 10.5% chromium; Cr oxide layer creates passive protection, which is why this steel group characteristics "stainless" or corrosion resistance. The ability of the oxide layer is self "healing - self healing", ie steel resistant to corrosion, even though the surface part continued worn. This is not true for carbon steel and low alloy often when they are protected from erosion by coating metals such as zinc or cadmium Cd Zn or inorganic coating such as paint.
Although all stainless steels depend on the presence of chromium, other metals are added to enhance their properties. The classification of stainless steel metal not by name but on the metallurgical structure (metallurgical structure) of them - the concept used to denote the arrangement of the atoms forming the grains, and they are concerned close to the magnification of the optical microscope through the polished form. Depending on the chemical composition of steel fixed, the structure may be austenitic or ferritic stable phases, mixed "duplex" of both, martenxit phase is formed when steel is cooled rapidly from high temperature, or chemical structure more efficient by the micro phase.
Relationship between differences of stainless steel they are divided according to Figure 1. A number of other comparable properties in their various steel are listed in Table 1.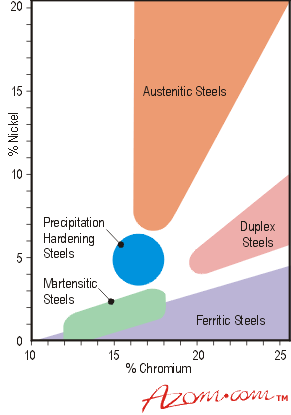
Figure 1. stainless steel.
Stainless steel austenitic steel group containing at least 16% Cr and 6% Ni (base steel grade 304 is specified as 18/8) and range depend on the high alloy or "super austenitic" as labels 904L with 6% Mo.
Additional elements such as Mo, Ti or Cu to change or improve their properties, making stainless steel adapted to specific applications in high temperature and corrosion resistance. Steel Group is also suitable for low temperature applications due to the influence of Ni content makes steel duochien avoid cold object at low temperature brittleness, Ni is the prime characteristic of this steel.
Relations between the various austenitic steels are shown in Figure 2. 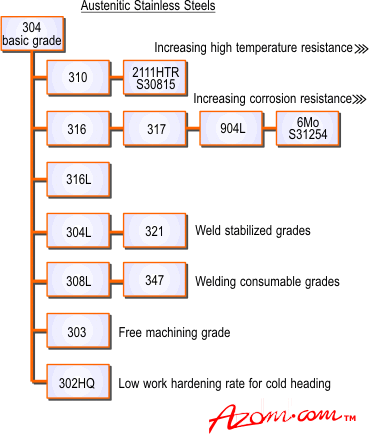
Figure 2. Stainless steel austenitic
Ferrite stainless steel These are only Cr steels is the main element in a proportion from 10.5 to 18%, as the steel grade 430 and 409. They are relatively corrosion resistance and poor fabrication, so to improve the quality of high-grade alloys are used as the 434 and 444 marks, and marks their Predominantly this is 3CR12.
Relations between the ferritic stainless steel grades is shown in Figure 3. 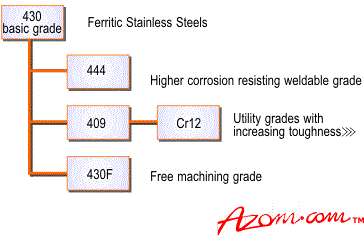
Figure 3. They ferritic steels.
Stainless steel martensite
Stainless steel is also macstenxit on the addition of chromium as the major alloying element but with a higher carbon content and lower chromium content (eg. 12% Cr in 410 and 416 grade) compared to ferritic steel type; Grade 431 has a chromium content of 16%, but its structure is still martensite despite this high chromium level and because this grade also contains 2% Ni.
Relations between the martensitic stainless steel is shown in Figure 4. 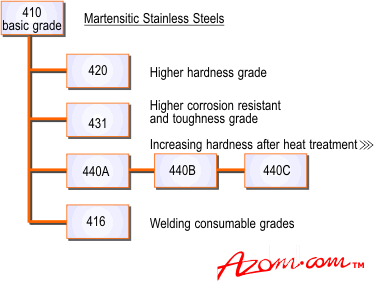
Figure 4. The martensitic stainless steel.
Duplex stainless steel
Duplex stainless steels such as 2304 and 2205 (these regulations, said composition Bi 4% and 22% Cr, Ni is 5% but both grades contain added the other alloying elements) have profiles constituted by a mixture autenite and ferrite. Duplex ferritic - austenitic steels combine some of the characteristics of each group: they are resistant to stress corrosion, albeit not quite as high as the ferritic steels; with toughness (toughness) outperformed the ferritic stainless steel and inferior to austenitic steels, and austenitic steels greater reliability the (annealed), with levels of about 2 times. In addition, duplex stainless steel with corrosion resistance equivalent to or better than 304 and 316, and generally hole corrosion (pitting corrosion resistance) than 316. They were reduced toughness at temperature -50 ° C and lower than after to heat (exposure) on the 300C, so we only used within the temperature limits of this.
Relations between them duplex stainless steel grade is shown in Figure 5. 
Figure 5. The duplex stainless steel.
Stainless Steel Hardening
The steel contains chromium and nickel to obtain high tensile strength. The most common grade is 630 marks in the "17-4 PH", with a 17% Cr, 4% Ni, 4% Cu and 0.3% Nb. The biggest advantage of this steel they can "handle" it. That is the process. Following processing, forming, ... steel can be hardened by heat treatment "aging" one step at a relatively low temperature, without causing fluctuations in the size of the product.
Characteristics of Stainless Steel
Features stainless steel groups can be seen in view of their comparable steel C "software" often. In terms of the most common, stainless steel:
Forged hardened high speed (hgher work hardening rate)
Higher viscosity (hgher ductility)
Hardness and higher reliability (Higher strength and hardness)
Reliability hot higher (Higher hot strength)
Higher corrosion resistant (corrosion resistance Higher)
Toughness at low temperatures better (Higher cryogenic toughness)
Reaction from inferior (only with austenitic steel) (Lower magnetic response (austenitic only))
Must retain corrosion resistant surface in the finished product.
These properties that are actually correct to austenitic steel and can change quite a lot for their steel and other steel.
These properties that are related to the field of application of stainless steel, but also influenced by the device and method of fabrication.
Table 1 (Part A). Comparative Properties of stainless steel families.
|
Alloy Group
|
Magnetism1
|
Forged hardening speed |
Corrosion resistance 2 |
Hardening ability |
|
Austenit
|
Không
|
Rất cao
|
Cao
|
Rèn nguội
|
|
Duplex
|
Có
|
Trung bình
|
Rất cao
|
Không
|
|
Ferrit
|
Có
|
Trung bình
|
Trung bình
|
Không
|
|
Martensit
|
Có
|
Trung bình
|
Trung bình
|
Tôi & Ram
|
|
Hóa bền tiết pha
|
Có
|
Trung bình
|
Trung bình
|
Hóa già
|
Table 1 (Part B). Comparative Properties of stainless steel families.
|
Nhóm hợp kim
|
Tính dẻo
|
Làm việc ở nhiệt độ cao
|
Làm việc ở nhiệt độ thấp 3
|
Tính hàn
|
|
Austenit
|
Rất cao
|
Rất cao
|
Rất tốt
|
Rất cao
|
|
Duplex
|
Trung bình
|
Thấp
|
Trung bình
|
Cao
|
|
Ferrit
|
Trung bình
|
Cao
|
Thấp
|
Thấp
|
|
Martensit
|
Thấp
|
Thấp
|
Thấp
|
Thấp
|
|
Hóa bền tiết pha
|
Trung bình
|
Thấp
|
Thấp
|
Cáo
|
Related Articles
- Inox in energy and electricity
- Stainless steel manufacturing industry chemistry
- Stainless steel water maintain integrity at Palomar California hospital
- Stainless Steel - 100 year history
- Stainless Steel Sheets
- Inox world
- Inox News
- CORROSION RESISTANCE INOX 304, 304L
- Learn about stainless steel Inox
- Comparison of 201 and 304 stainless steel













